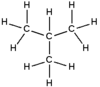
Isobutane, also known as methylpropane or 2-methylpropane, is an alkane, isomeric with butane. It is the simplest alkane with a tertiary carbon. Recent concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers, and as a propellant in aerosol sprays. When used as a refrigerant or a propellant, isobutane is also known as R-600a. Some portable camp stoves use a mixture of isobutane with propane, usually 80:20. Isobutane is used as a feedstock in the petrochemical industry, for example in the synthesis of isooctane.
Its UN number (for hazardous substances see shipping) is UN 1969. Isobutane is the R group for the amino acid leucine.
Isobutane is the trivial name retained by the International Union of Pure and Applied Chemistry (IUPAC) in its 1993 Recommendations for the Nomenclature of Organic Chemistry
Methylpropane is the systematic name. The substituent number (2-) is unnecessary because there is no isomer of this molecule with methylpropane as part of its name.
Kullanımı
Soğutucu olarak;
Blends of pure, dry "isobutane" (R-600a) (commercial term used to describe isobutane mixtures) have negligible ozone depletion potential and very low Global Warming Potential (having a value of 3.3 times the GWP of carbon dioxide) and can serve as a functional replacement for R-12, R-22, R-134a, and other chlorofluorocarbon or hydrofluorocarbon refrigerants in conventional stationary refrigeration and air conditioning systems.
As a propellant for aerosol cans and foam products.
Reports surfaced in late 2009 suggesting the use of isobutane as a refrigerant in domestic refrigerators was potentially dangerous. Several explosions resulting from the isobutane leaking into the refrigerator cabinet and a spark from the electrical system have been reported in the United Kingdom. Although unclear how serious this could be, at the time this report came out it was estimated 300 million refrigerators worldwide use isobutane as a refrigerant.
Although these stories are only speculation , the use of a flammable gas as a refrigerant is quite dangerous and encompasses a great deal of risk. The normal risks a CFC or other toxic refrigerant would have when it escapes, are mainly related to depletion of breathable air and frosting at the point of escape. Isobutane has an explosion risk associated also (in addition to depletion of breathable air and frosting). This explosion risk is more dangerous directly to anyone in the vicinity should it accumulate and come into contact with any ignition source.
İsobütan Gazı için kullanılabilecek Gaz Dedektörleri
- Smart3G C2
- Smart3G D2

